FDA Approves External Pump, AspireAssist, To Shed Weight Among Obese
The Food and Drug Administration (FDA) Tuesday approved an external pump to help dump part of stomach content into the toilet.
Dubbed as “assisted bulimia,” the new and unusual weight loss device has been approved for use in very obese patients. It is claimed to shed more than 12 percent of body weight, resulting far more than most diets and most pills.
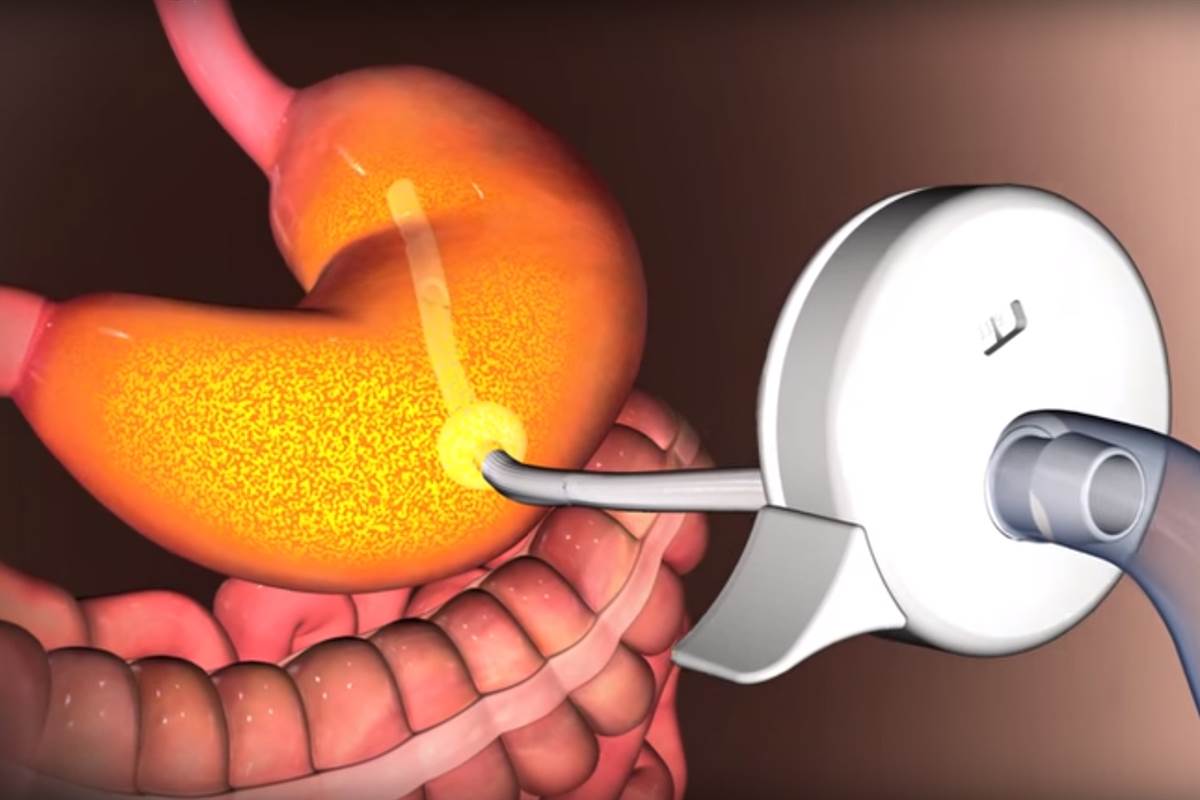
The device induces a tube in the stomach to a port outside of the abdomen and helps in removing third of the content of stomach at a time.
Manufactured by Aspire Bariatrics of King of Prussia in Pennsylvania, the device has resulted with lost of about 46 pounds in trials during the first year. In the second year the weight loss increased to 50 pounds.
In a released statement the FDA said, “The AspireAssist device should not be used on patients with eating disorders, and it is not intended to be used for short durations in those who are moderately overweight.”
Guidelines suggest the device is made for patients aged 22 and above and having a body mass index of 35 to 55.
Usually diet drugs fail in shedding weight and the AspireAssist approach is believed to help in providing effective control of calorie absorption.
As of now the manufacturer has not priced tagged the device.
Those people are considered obese whose body mass index (a measure of height to weight) hits 25. Obesity is calculated using BMI.